So we all hang on this as a prognosticator, as well as a gauge of the state of our patients both on the unit and prior to coming up to us. But should we be focussing on Lactate as much as we think we should? Is it the ‘magic bullet’ to adequate resuscitation?
A bit of a recap on those awful metabolic pathways!
Lactate is produced by most tissues in the human body, with the highest level of production found in muscle. Under normal conditions, lactate is rapidly cleared by the liver with a small amount of additional clearance by the kidneys. In aerobic conditions, pyruvate is produced via glycolysis and then enters the Krebs cycle, largely bypassing the production of lactate. Under anaerobic conditions, lactate is an end product of glycolysis and feeds into the Cori cycle as a substrate for gluconeogenesis (see the cartoon below). Lactate exists in two forms isomers: L-lactate and D-lactate. It is the L-isomer we are interested in. It is important to note that oxygen shortage per se does not lead to hyperlactataemia, rather increasing energy requirements not met by adequate supply.
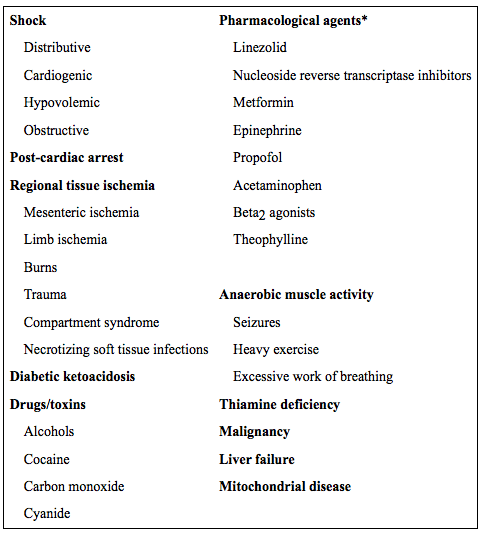
The terms lactate and lactic acid are often used interchangeably but lactate (the component measured in blood) is strictly a weak base whereas lactic acid is the corresponding acid. “Lactic acidosis” is often used clinically to describe elevated lactate but should be reserved for cases where there is a corresponding acidosis (pH < 7.35). The exact pathophysiology of elevated lactate in various conditions is likely multifactorial, patient-specific, and disease-specific. In general, lactate elevation may be caused by increased production, decreased clearance, or a combination of both. Contributing factors appear to include: hypoperfusion due to macro- and/or microcirculatory dysfunction, mitochondrial dysfunction (including potential lack of key enzymatic co-factors) and the presence of a hypermetabolic state, among others. Causes of elevated lactate are shown below
- Under stable conditions glucose is converted to pyruvate, generating 2 ATP. Pyruvate is then subsequently fully oxidized to CO2 generating ~36 ATP.
- Under stress, glycolysis can increase by a factor 100 to 1,000, provided that glucose is present and pyruvate is converted to lactate. Irrespective of optimal mitochondrial function and oxygenation, such a rate of pyruvate production will saturate the mitochondrial tricarboxylic acid cycle and oxidative phosphorylation.
- During recovery, lactate is converted back to pyruvate and fully oxidised.


(See also video on Lactate in Sepsis and lactate physiology in our video resources section)
The metabolism of glucose during tissue hypoxia results in the production of [lactate]-, ATP, and water. The production of H+ originates from the hydrolysis of ATP to ADP. In the presence of oxygen and provided that OxPhos can keep up with glycolysis, these H+ ions can be used together with lactate in the OxPhos in the mitochondria and acidosis is thus less likely to develop. But…even at higher lactate levels, there is only a weak correlation with low arterial pH. When evaluating the significance for patient outcome and the origin of the metabolic acidosis, it is probably more realistic to use the term: lactate-associated metabolic acidosis, a combination that also carries the highest risk of mortality.
Studies have discussed the fact that blood lactate concentrations have an established prognostic value in circulatory shock or after cardiac arrest, their relationship with morbidity and length of stay in general intensive care unit (ICU) populations has not been well defined however.
Some common myths to dispel while we are at it…infusion of Hartmanns does not hamper the accuracy of lactate measurement and interestingly, renal replacement therapy eliminates only negligible amounts of lactate. But, using lactate-containing buffer solutions can induce transient hyperlactataemia.
Under normal physiological circumstances, we are extremely adept at clearing large lactate loads. This is noted in particular after ROSC in cardiac arrest, as well as the rapid decrease we see in lactate levels following exercise. Likewise, the body is equally as good at clearing very large exogenous loads of lactate during high-volume continuous veno-venous hemofiltration. Liver dysfunction has been shown to impair lactate clearance quite significantly, which is why this organ failing in amongst your multi-organ failure picture has such profound metabolic consequences, particularly when fuelled by hypo perfusion.
In sepsis, increased glucose metabolism (and thus lactate production) occurs. As well as microcirculatory impairment, we also get major impairment in the microvascular circulation. This is the vital component in getting vital nutrients and oxygen to our vital organs and flushing waste products of metabolism out. It is this, added to the fact that the rate limiting enzyme pyruvate kinases is also inhibited, that we see a ‘triple whammy’ effect. Lactic acid levels have become a useful marker for tissue hypoperfusion and may also serve as an endpoint for resuscitation in patients with sepsis. Drugs stimulating pyruvate kinase have been trialled, but to no good effect.

Do we chase it to resuscitate then..?
We always chase MAP, as we assume that it has a strong relationship to the occurrence of inadequate tissue oxygenation. It also has a long-time established relationship with morbidity and mortality. Lactate levels could represent a useful goal of initial resuscitation in many clinical conditions.
So, effective lactate clearance has been associated with decreased mortality in a number of settings and conditions. Failure to clear lactate portends to a worse outcome. In patients with presumed tissue hypoperfusion (for example, septic shock), failure to clear lactate should prompt reassessment of the resuscitation effort. Persistent lactate elevation may indicate unrecognized ischemic bowel, an uncontrolled source of infection, inadequate flow (either from inadequate intravascular volume or inadequate cardiac contractility), concomitant pharmacologic insult (e.g., associated metformin-induced mitochondrial injury in a septic patient with renal failure), unrecognized thiamine deficiency, irreversible mitochondrial injury or other problems.. Continual reassessment for unrecognized causes is therefore warranted in cases of persistent elevation, as treatment may have to be tailored accordingly.
Point in time measurements as well as the area under the curve of increased lactate levels have been shown to relate to both morbidity (organ failure) and mortality in different patient groups. In the early phase of resuscitation, lactate levels seem to be more closely related to outcome than frequently used haemodynamics, oxygen delivery or oxygen consumption. Therefore, it may be more appropriate to look at multiple parameters in early resuscitation. Hypoxia and lactate levels are undoubtedly strongly linked, but lactate levels per se, are governed by many more metabolic processes and thus are subject to many disturbances found in various clinical situations.
What do the studies tell us?
Until recently, the only known single-centre clinical trial advocating such lactate-directed therapy was performed in postcardiac surgery patients. This study showed a reduction in morbidity but was not powered to study the effect on mortality. It’s difficult though to translate these findings to a more general and frequently much sicker population on ITU.
A trial in the ED randomised 300 septic patients to a lactate clearance (≥10%) or a central venous oxygen saturation (ScVO2 ≥70%), as goals of early resuscitation. The intervention lasted until either all goals were achieved or 6 hours after start of the study. There were no differences in treatments administered during the initial 72 hours of hospitalization. In-hospital mortality in the lactate group was non-inferior to the ScVO2 group (but you can argue that both lactate levels and ScVO2 are equally effective as goals of therapy).
It is questionable whether a 10% reduction in lactate in 6 hours represents effective resuscitation. For example, this would have equated to a reduction in lactate from 5.0 mmol/L to 4.5 mmol/L after 6 hours of treatment. Not sure I would be entirely happy with that in any of my patients! Even those that survive resus attempts after cardiac arrest often get up to a 30% reduction in lactate in the first hour. It is more likely that we should be focussing on the trend in decrease / increase in lactate as our guide to resuscitation. Moreover, the failure to decrease lactate levels at all in response to treatment has more implications for prognosis, and is often a real situation we face when all we do is still failing.
In a multicentre, open-label, randomized, controlled trial, 348 patients were randomly allocated to either lactate-guided treatment (lactate group) or nonlactate-guided treatment (control group) during the first 8 hours of ICU stay.
- Lactate arm – goals were a 20% or more decrease in lactate levels per 2 hours and the normalization of ScvO2 (>70%).
- Control arm – lactate levels were not available to the treating physicians during the first 8 hours except for the admission level required for randomization.
Patients in the lactate clearance group had shorter time in the intensive care unit, were weaned faster from mechanical ventilation and also inotropes. There was no difference in actual lactate clearance between the groups and no difference in mortality before adjusting for risk factors. When adjusting for predefined risk factors there was a significant decrease in hospital mortality (hazard ratio 0.61; confidence interval 0.43–0.87).
45% had hyperlactataemia and compared to those with normal lactates, these patients had:
- Higher APACHE II scores (13.3 +/- 6.9 vs 10.0 +/- 5.2)
- Higher SOFA scores, (5.3 +/- 3.3 vs 3.3 +/- 2.3)
- No difference in ICU stay (LOS)
- A mortality 23% Vs 9%
Mortality rates seen were:
- 17% in patients with lactate concentrations from 2-4 mEq/l
- 64% in those with concentrations more than 8 mEq/l.
- Non-survivors had higher lactate concentrations than survivors on admission, and after 24 and 48 hours.
Risk factors for developing hyperlactataemia, present on admission were:
- SOFA score > 5
- mean arterial blood pressure less than 70 mmHg
- blood sugar greater than 110 mg/dl
- current use of vasopressors.
So…this showed a direct relationship between the serum lactate level on ICU admission and not only the risk of death in ICU but also the length of ICU stay. A good morbidity predictor, certainly.
Another large study of 1278 patients being admitted with infection found that lactate levels could correctly stratify patients according to mortality.
- Lactate levels of
- 0–2.4 had mortality of 4.9%
- 2.5–3.9 had a mortality of 9%
- ≥4 mmol/L had a mortality of 28.4%
Furthermore, evaluation of lactate clearance through serial measurements has been shown to be a useful predictor of morbidity and mortality. Patients who clear an initially elevated lactate level to < 2.5 mmol/L or < 4.0 mmol/L within 24 hours have significantly better outcomes than patients whose elevated lactate persists. Serial lactate measurements may be useful in documenting treatment response to various therapeutic interventions.
Vasodilate them then…
An important addition to the protocol treatment was the administration of a vasodilator when ScvO2 levels where normal but lactate levels did not decrease sufficiently. This is the first study to address this important problem in the resuscitation of critically ill patients, because normalisation of ScvO2 is generally regarded as a restoration of the balance between oxygen delivery and oxygen demand that should result in normalization of lactate levels. As mentioned above, the micro circulation in septic patients is often compromised and it is this that impairs lactate clearance. Even when O2 demand:supply relationships have been optimised, we see no adequate decrease in lactate levels in overtly septic subjects. Hence the addition of a drug that was thought to improve this situation, but it may only do so when this situation is existent…not in normal patients!
Like the Rivers landmark study, protocol patients received significantly more fluids during the intervention period and far less fluid during the subsequent observation period. With this and the treatment with vasodilators,there was an almost statistically significant (p = 0.067), 20% relative reduction in mortality in addition to a strong statistically significant reduction in morbidity (duration of mechanical ventilation and ICU stay, p = 0.006).
In a study by Boerma et al, nitroglycerin administration was used in addition to a standard resuscitation protocol in all patients in the protocol group. This study showed no differences in the microcirculation between the two groups and a trend towards an increased mortality in the protocol group. In their study, nitroglycerin was also used in patients with normal microcirculation, decreasing, or even normal lactate levels, etc. This is why its addition is not warranted in normal resuscitation.
My conclusion on all of this…
- Lactate certainly has a place in risk stratification of the criticaly ill
- Increasing lactate levels usually correlate with increased morbidity and mortality.
- Lactate production does not simply result from anaerobic metabolism. Changes in aerobic mechanisms and changes in the body’s ability to clear lactate also play a role.
- Lactate may be an epiphenomenon of disease severity
- High lactate may give warning that patients who are not improving or deteriorating in the presence of stable haemodynamic parameters may need additional diagnostic and therapeutic interventions.
- Lactate-directed resuscitation therapy has clinical benefit for critically ill patients, although the exact mechanism behind it remains uncertain.
- Lactate measurement probably should be accompanied by venous saturation monitoring to guide decision-making and therapy.
LACTATE MONITORING (TRENDING) IS A VALUABLE PARAMETER IN THE EARLY RESUSCITATION OF THE CRITICALLY ILL PATIENT – JW Oct 2016




















Leave a Reply